

Rifampicin resistant (RR)-TB is estimated to cause 13% of all antimicrobial resistance-attributable deaths worldwide and is driven by both resistance acquisition and person-to-person transmission




Antimicrobial stewardship programs are crucial in tuberculosis (TB) management due to the escalating threat of drug resistance and the need to optimize the use of existing, and potentially new, anti-TB drugs. Currently, there is no evidence-based regimen for patients with Rifampicin resistant (RR)-TB and bedaquilin resistance (BDQr).
Thus, treatment becomes extremely challenging, implies toxic regimens with duration exceeding 18months, showing unsatisfactory success rates, consequently risking acquired resistance to other drugs in the regimen and further transmission of the highly resistant TB strains.
How to treat BDQr/RR-TB with the current arsenal of TB drugs is unknown.




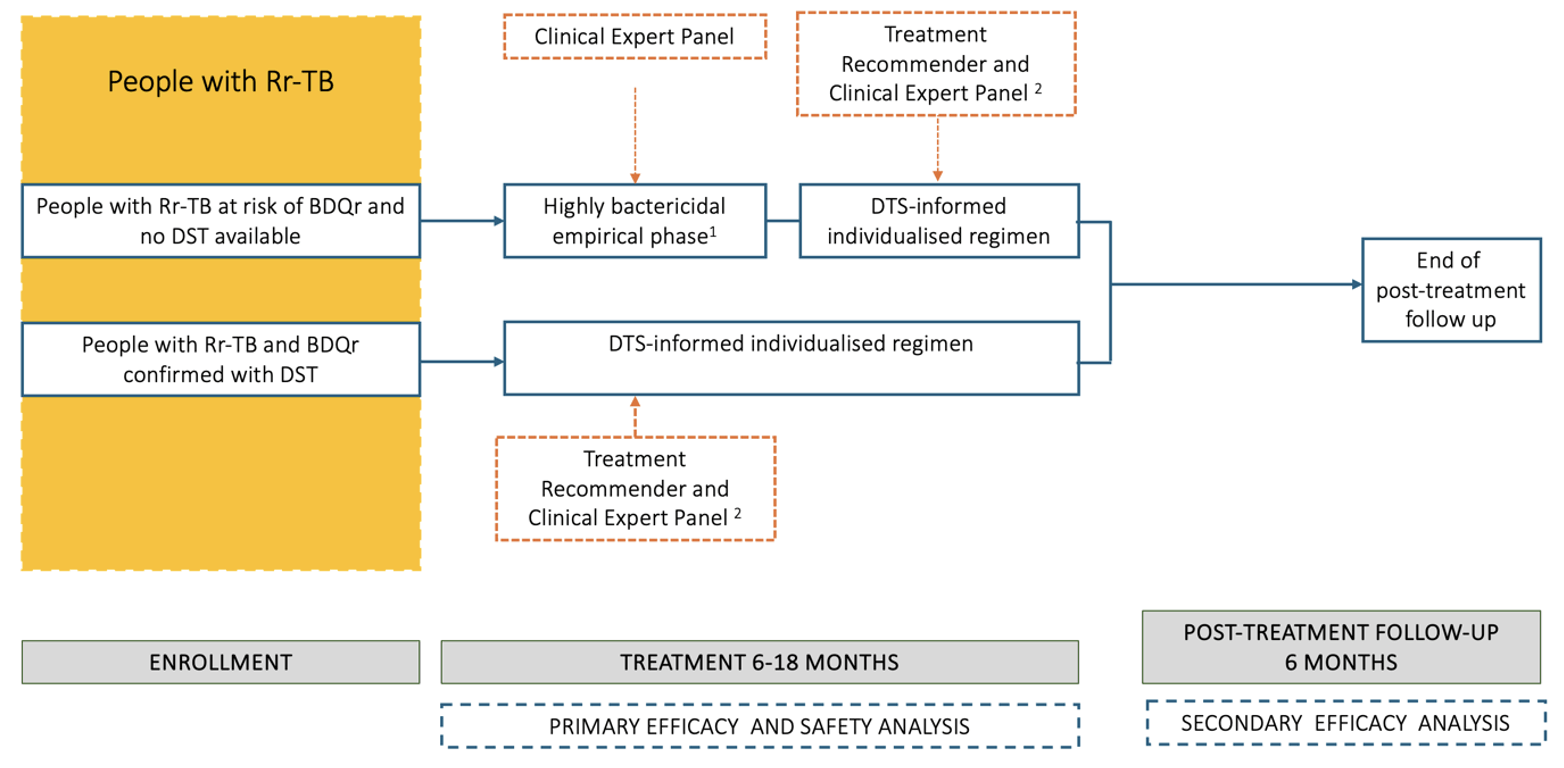
Aims to rapidly reduce the bacillary load in patients with TB at risk or with Bedaquilin-resistance (BDQr) and with Rifampicin-resistance (RR-TB) by using an empirical highly bactericidal first treatment phase. This first phase will be followed by a drug-susceptibility test-informed, individualised and AI-supported treatment regimen. Participants will be enrolled in high burden, (relatively) low resourced areas within Nigeria, South-Africa and Mozambique. Baseline and acquired resistance will be monitored and TASP will determine if low level BDQr can be overcome with a high dose BDQ-containing regimen. Safety of all treatment intervention components will be monitored rigorously.
WP1 will assess treatment success of a treatment regimen for TB resistant to rifampicin and bedaquiline using new combinations and high(er) drug dosages of existing antimicrobials.
Stepwise approach to regimen construction
1/ Recycle BPaL drugs and add clofazimine, for which there is no rapid DST
BDQ : at high dose (see above), the other drugs at normal dose.
(at the end of this step, a max of 4 drugs will be included: B Pa L Cf)
2/ Apply results Xpert XDR results:
● Use high-dose Lfx (see above) unless resistance on Xpert MTB/XDR is documented; also use high-dose Lfx if Xpert XDR shows gyrA D94A or gyrA A90G as mutations, as these usually confer low-level resistance.
● Use Am (dose see above) unless Xpert MTB/XDR shows resistance. Also use Am if Xpert XDR shows eis MUT without rrs MUT.
(at the end of this step, a max of 6 drugs will be included: Am-BPaLLfCf)
3/ Add a carbapenem
● If the participant experienced treatment failure while on BPaL(M/L) or any of the previous drugs BDQh, LZD, Pa or FQ or if those drugs cannot be used due to intolerance or are not available.
(at the end of this step, a max of 7 drugs will be included: Cbm Am B Pa Lz Lf Cf; if less than 7 drugs, proceed to step 4)
4/ In case after step 3 the regimen did not yet contain 7 drugs, add drugs until the regimen is composed of 7 drugs
● Add pyrazinamide if clofazimine was not used, and then use delamanid instead of Pa
● Add drugs, in order of preference: isoniazid high-dose (unless Xpert XDR shows both InhA and KatG mutations), ethionamide (unless Xpert XDR shows InhA mutations), cycloserin, PAS
Lead partner: Aurum Institute
Participating partners: Fundaçao Aurum, University of Ibadan, Lagos State University, University of Rwanda, CNHPP, University of Cape Town, Uppsala University, Institute Tropical Medicine, Vrije Universiteit Amsterdam
Evidence-based, AI-aided, regimen construction guide for RR-TB with BDQr
Work package 2 will integrate thin layer agar (TLA) for drug susceptibility testing (DST) and minimal inhibitory concentration (MIC) determination into the diagnostic algorithms of the recruiting countries. TLA testing will be conducted in parallel with WHO-recommended methods, including culture and DST in MGIT liquid medium, broth microdilution (BMD), and targeted next-generation sequencing.
This work package constitutes two phases:
-The initial phase involves establishing BMD and TLA at the Benin Reference Center, CNHPP. This will be followed by an assessment to identify the laboratories responsible for testing samples from TASP participants. With the support of Rwanda's National Reference Lab and ITM's Supranational Reference Lab, TLA will be implemented in at least one laboratory in each recruiting country. Staff at each site will be trained on this technique. All procedures for DST and MIC reference methods will be assessed for quality and compliance with standardized procedures, to ensure consistency across laboratories. After implementation, expected to be completed by May 2026, the routine phase will start. During this phase, the performance of all tests will be closely monitored. Any discrepancies observed on-site between WHO-recommended methods will undergo further investigation through whole genome sequencing (WGS) at ITM, which will provide a final result for comparison with TLA.
Lead partner: University of Rwanda
Participating partners: CNHPP, Institute Tropical Medicine, University of Ibadan, Lagos State University, Aurum Institute, Fundaçao Aurum
WP3 aims to evaluate the early bactericidal activity (EBA) and pharmacokinetics (PK) of high-dose amikacin, bedaquiline, and levofloxacin in rifampicin-resistant TB patients at risk of bedaquiline resistance. At trial initiation, complete drug susceptibility testing (DST) results are unavailable for these patients. The empirical intensive treatment approach targets these patients since they have limited therapeutic options.
We will assess early drug efficacy by measuring the decline in viable mycobacterial burden over the first two weeks of treatment. Time to positivity (TTP) serves as an indirect marker of bacterial load, with shorter TTP indicating a higher burden. These data will be used in mechanistic mathematical models to estimate bacterial kill rates, capture inter-patient variability, and compare the EBA profiles of high-dose regimens with appropriate controls.
PK profiles of high-dose bedaquiline will be compared directly to the standard-dose BPaLM regimen (bedaquiline, pretomanid, linezolid, and moxifloxacin), while amikacin and levofloxacin PK data will be interpreted in relation to historical controls.
Together, these sub-studies will inform dose optimization, support the safe and effective use of high-dose regimens, and guide empirical treatment strategies for rifampicin-resistant TB patients with incomplete DST and a heightened risk of further resistance development.
Lead partner: University of Cape Town
Participating partners: Uppsala University, Institute Tropical Medicine, Aurum Institute
While Infection Prevention and Control (IPC) in health care has had a strong emphasis on fomites, hand hygiene, universal precautions, and sterilization, less attention has been paid to the airscape, in part because the prominence of aerosol route of transmission of many AMR pathogens has only recently been clarified. African health care facilities need air filtration, purification, ventilation solutions that are simple, inexpensive tools, and are not highly dependent upon clinician or patient behaviour. Combining low-cost materials found locally, we will build, deploy, and test how well these simple portable air cleaners reduce the concentration of particulates in indoor air and offer insights into what and how much infectious agents can be found in health facility air.
We have 3 broad scopes of work in TASP work package 4:
Study 1: Low-cost bioaerosol capture, dust retrieval, and DNA extraction
Study 2: Low-cost portable Air Cleaner (PAC) efficacy trial
Study 3: Additionality of filter forensics and PACs for AMR Surveillance
Lead partner: Institute Tropical Medicine
Participating partners: University of Toronto, Fundaçao Aurum, Aurum Institute, University of Ibadan, Lagos State University
Work Package 5, coordinated by Vrije Universiteit Amsterdam, focusses on the economic evaluation of the TASP intervention. We will collect detailed information on the cost of the TASP intervention (e.g. clinic visits, number and type of laboratory tests, medication) and on additional costs incurred by patients (e.g travel, out-of-pocket payments). Combining this information with the results of the clinical trial and the cost of conventional treatment regimes, we assess the cost-effectiveness of the TASP intervention by estimating the additional cost for each increase in TB-free survival.
Lead partner: Vrije Universiteit (VU) Amsterdam
01 June 2025
19 September 2025
16 Oktober 2025
We are pleased to announce the search for a PhD student (4-year, fully funded). This position will be based in Mozambique.
We are looking for a fluent Portuguese speaker and we have several possible profiles in mind. The training budget of the PhD can fill gaps in any the person's AMR IPC, TB background.
Interested? See the full-position info here.
Visão geral: Limpeza de Ar Portátil de Baixo Custo e Vigilância da Resistência Antimicrobiana em Hospitais Africanos
Descrição do Projeto Este projecto de doutoramento aborda a necessidade urgente de soluções escaláveis de prevenção de infecções e monitorização de RAM em ambientes de saúde com recursos limitados.
estudante de doutoramento será o coração da equipa que:
• Projectará e avaliará unidades de filtragem de ar portáteis e de baixo custo, fabricadas com materiais de origem local em Moçambique, África do Sul e Nigéria.
• Desenvolverá e validará novos métodos para a vigilância de RAM em ambientes clínicos e hospitalares em Moçambique, África do Sul e Nigéria.
• Irá conceber e testar abordagens forenses quantitativas de filtragem para caracterizar agentes patogénicos transportados pelo ar e genes de resistência.
• Colaborará com parceiros de engenharia, microbiologia, epidemiologia e saúde pública na Europa (Instituto de Medicina Tropical (BE), Vrij Universiteit (NL) e no Canadá (Universidade de Toronto) para desenvolver e aplicar ferramentas e métodos de ponta.
Procuramos um investigador em início de carreira, apaixonado, autodirigido e colaborativo, com o seguinte perfil:
✓ Mestrado (ou equivalente) em saúde pública, engenharia, microbiologia, epidemiologia, controlo de infecções transmitidas pelo ar, saúde ambiental ou ciências ambientais. ✓ Fortes competências de comunicação e colaboração, especialmente em ambientes com múltiplos fusos horários, multigeracionais e multiculturais.
✓ Curiosidade, iniciativa e competências demonstradas para a resolução de problemas.
✓ Disponibilidade e capacidade para viajar frequentemente (até 30%) entre Lagos, Maputo e Joanesburgo.
✓ Disponibilidade para viajar anualmente para Antwerp/Amsterdam
✓ Fluência em Português (B2)
✓ Proficiência em Inglês (C2)
✓ Nacionalidade Moçambicana (Sem obrigatoriedade de residir em Moçambique, pode estar actualmente a trabalhar ou estudar no estrangeiro)
✓ Se estiver empregado numa organização de investigação e desejar manter o seu cargo durante o doutoramento, o seu empregador deve estar disposto a conceder-lhe permissão para trabalhar pelo menos 80% do tempo na sua investigação de doutoramento durante 4 anos.

No Code Website Builder